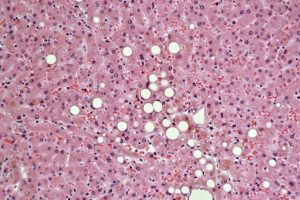
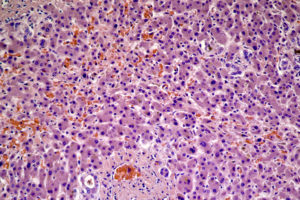
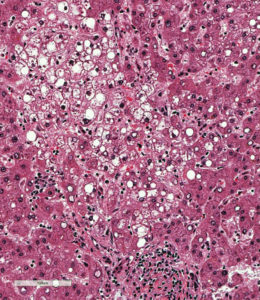
NASH Samples – Nonalcoholic Steatohepatitis
Table of Contents
Introduction | Treatments and Standard of Care | Considerations for NASH Researchers | References
If you use NASH, NAFLD, or normal liver samples in your research, contact us with your procurement services questions.
Introduction
The ability to procure tissue specimens for research, as well as the availability of any pathology data, medical history data, or results of testing for biomarkers, will always be limited by the prevalence of a condition, the course of development of the disease, and the standard clinical practices used in the diagnosis and treatment of the pathology. For scientists working to examine the chronic liver indications Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH), an understanding of standards of care is recommended to inform the design of research studies.
A Note on Diagnosis
NASH can only be definitively diagnosed by histological evaluation through biopsy, provided that all other criteria are met. Indications that a patient may have nonalcoholic fatty liver disease (NAFLD) or NASH in particular include evidence of hepatomegaly, abnormal liver function test results, and evidence of hepatic steatosis as detected by imaging tests (CT, MR, or ultrasound) (Reid, 2001). What distinguishes NASH from NAFLD and other liver diseases is the presence of hepatic steatosis and inflammation with hepatocyte ballooning, with or without fibrosis. Liver biopsy, however, is recommended only for those who could benefit diagnostically or prognostically from one (Chalasani, et al. 2012). A liver biopsy will enable a physician not only to determine whether NASH is present in a patient, but also the degree or grade of steatosis and fibrosis. Additionally, it is not unusual for diagnosis to be somewhat ambiguous with samples from the same live sometimes showing signs of NAFLD, NASH, and fibrosis from other causes.
Treatments and Standard of Care in U.S.
Lifestyle Intervention
Because NASH is usually tied to obesity, poor diet, and inactivity, weight loss and exercise are almost always going to be primary recommended goals for patients with NASH. Lifestyle intervention is also important because type 2 diabetes mellitus and metabolic syndrome are common in patients with NASH (Gitto, et al., 2015). Weight loss of about 3-5% of body weight appears to decrease hepatic steatosis and improve metabolic parameters, although not substantially (Chalasani, et al. 2012). Weight loss of 7% or more has been shown to significantly decrease steatosis, improve necroinflammation, and lessen the overall disease state (Promrat, et al., 2010; Musso, et al., 2012). Gradual weight loss is recommended, however, because abrupt weight loss could potentially worsen the injury to the liver (Gitto, et al., 2015). Regardless, patient noncompliance is a common issue, and a large proportion of patients with NASH do not achieve their weight loss and exercise goals (Musso, et al., 2012).
Drugs/Pharmaceuticals
Two commonly prescribed drugs that studies have shown to have some effect in improving the disease state are vitamin E (RRR-α-tocopherol) and pioglitazone. Vitamin E is an antioxidant that has a positive effect on the level of transaminases, liver inflammation, and fat accumulation (Gitto, et al., 2015). Vitamin E is not recommended for patients with diabetes or cirrhosis, however, and it also has no proven effect on long-term morbidity and mortality or on fibrosis (Chalasani, et al. 2012). A recommended daily dose of vitamin E is 800 IU/day (Chalasani, et al. 2012). This is in keeping with a study by Sanyal, et al. in 2010 in which 84 adults with NASH but without diabetes were prescribed 800 IU/day of vitamin E for 96 weeks. Significant improvements in steatosis, inflammation, and hepatocellular ballooning were noted in the vitamin E recipients as compared with the placebo recipients (83 subjects). Pioglitazone also has a positive effect on transaminases, liver inflammation, and fat accumulation, but it has a negative impact on weight (Sanyal, et al., 2010; Gitto, et al., 2015).
Surgical/Transplantation
A minority of patients with NASH (fewer than 20%) will experience or show progression to cirrhosis and end-stage liver disease (Reid, 2001). For those who do have livers in such poor condition, transplantation is required. Because the incidence of NAFLD—and NASH, in consequence—is on the rise, NASH is now the third most common cause for liver transplantation in the United States, and will probably become the most common cause for liver transplantation within the next ten years. From 2001 to 2009 alone, the percentage of liver transplants attributed to NASH increased from 1.2% to 9.7% (Charlton, et. al., 2011).
Considerations for NASH Researchers
Since NASH is not treated surgically as part of US standard care, researchers will not be able to find usable portions of NASH liver removed from living patients, with the exception of cases severe enough to require transplantation. Very few clinics perform biopsies on NASH or NAFLD, but in the limited number of instances when a biopsy is performed, the entire tissue sample is typically exhausted in the diagnostic procedures.
Thus, NASH specimens available to researchers come almost entirely from either 1) liver biopsies 2) liver explants (livers with NASH that have been removed from patients in the process of transplantation) or 3) liver samples that were collected postmortem.
Liver samples from explants
Liver explants are difficult to obtain, but the number of clinics willing to attempt this procedure is growing. Because the liver is a large organ, each explant will yield a significant number of FFPE and frozen tissue samples. Lab-Ally does have access to good numbers of explant cases and these samples are often well annotated with good clinical information and patient history often available, but unfortunately explant material is expensive.
Postmortem liver samples
Liver samples from postmortem donors, that do not show evidence NASH, can be used as normal controls. However, such samples are rarely completely normal, as the donors are often older, and livers from older individuals may show signs of fibrosis, hemochromatosis (accumulation of iron) or related indications like ASH. “True” normal liver samples from young and healthy donors are hard to find since such individuals don’t typically die or require liver transplants. Postmortem liver samples that show evidence of NASH can sometimes be collected even if the donor’s medical history does not mention the disease by examining material from donors known to be obese. Unfortunately little or no useful clinical information is available for these types of cases.
Another challenge with obtaining liver specimens postmortem is that liver tissue often degrades rapidly after death as the liver is full of enzymes that can cause hemolysis/autolysis. Thus, low postmortem intervals (PMIs) and good quality control by a pathologist are extremely important to produce high quality liver samples that can be used by researchers. Lab-Ally has a world class pathologist on-site who checks all liver specimens before shipment to researchers in order to ensure excellent quality control.
References
- Reid, A. E. (2001). Nonalcoholic Steatohepatitis. Gastroenterology, 121(3), 710–723. http://dx.doi.org/10.1053/gast.2001.27126
- Gitto, S., Vitale, G., Villa, E., and Andreone, P. (2015). Treatment of Nonalcoholic Steatohepatitis in Adults: Present and Future. Gastroenterology Research and Practice. doi:10.1155/2015/732870
- Chalasani, N., Younossi, Z., Lavine, J. E., Diehl, A. M., Brunt, E. M., Cusi, K., Charlton, M. and Sanyal, A. J. (2012). The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology, 55: 2005–2023. doi:10.1002/hep.25762
- Promrat, K., Kleiner, D. E., Niemeier, H. M., Jackvony, E., Kearns, M., Wands, J. R., Fava, J. L. and Wing, R. R. (2010). Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology, 51: 121–129. doi:10.1002/hep.23276
- Musso, G., Cassader, M., Rosina, F., Gambino, R. (2012). Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetology, 55(885). doi:10.1007/s00125-011-2446-4
- Sanyal, A. J., Chalasani, N., Kowdley, K. V., McCullough, A., Diehl, A. M., Bass, N. M., … for the NASH CRN. (2010). Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. The New England Journal of Medicine, 362(18), 1675–1685. http://doi.org/10.1056/NEJMoa0907929
- Charlton, M. R., Burns, J. M., Pedersen, R. A., Watt, K. D., Heimbach, J. K., Dierkhising, R. A., (2011). Frequency and Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Gastroenterology, 141(4), http://dx.doi.org/10.1053/j.gastro.2011.06.061.