Pancreatic Cancer Samples
Table of Contents
Introduction | Classification | Diagnosis and Treatment | Considerations for Researchers | References
Looking for human pancreatic cancer samples for use in research? Contact us for a consultation.
Introduction
Pancreatic cancer is an aggressive cancer involving tissues of the pancreas, the organ that lies behind the lower regions of the stomach and is responsible for the secretion of enzymes involved in digestive metabolism of sugars (insulin, glucagon, and somatostatin) as well as protein (trypsin and chymotrypsin), carbohydrates (amylase), and fats (lipase). As such, pancreatic cancer can be a life-threatening disease that is difficult to treat and has a high mortality rate. Pancreatic cancer rarely strikes individuals under the age of 40, and more typically affects individuals over the age of 70 (Ryan, Hong, & Bardeesy, 2014). A recent study has predicted that by 2030 pancreatic cancer will be the second deadliest form of cancer in the United States (Rahib et al., 2014 – https://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=24840647).
Symptoms of Pancreatic Cancer
Symptoms of pancreatic cancer typically do not appear until the cancer is fairly advanced. Combined with its ability to spread rapidly, this result in a generally poor prognosis for patients with this disease. Symptoms, when they present, include (Hanada et al., 2014; Yamaguchi et al., 2014):
- Abdominal or back pain
- Unexplained weight loss
- Jaundice (yellow skin)
- Light-colored stool
- Dark urine
- Loss of appetite
Causes & Risk Factors
While no identifiable cause is found in most individuals that develop pancreatic cancer, there are several known risk factors, including (Edderkaoui & Eibl, 2014):
- Age of 60 or greater
- BRCA-1 or BRCA-2 mutations
- Tobacco use
- Birt-Hogg-Dubé syndrome (BHD)—associated with mutations in the FLCN gene.
- Obesity
- Sedentary/inactive lifestyle
- A history of diabetes
- Chronic pancreatic inflammation
- High-fat diet.
- Prior stomach surgery
- Chronic hepatitis B or H. pylori infection(s)
Classification of Pancreatic Cancer
Classification by Stages
Given the pancreas’ location within the abdomen, the stage of pancreatic cancer is typically identified via CT scan (De La Cruz, Young, & Ruffin, 2014). There are five stages of pancreatic cancer as defined by the American Joint Committee on Cancer (AJCC) TNM Staging system (Edge & American Joint Committee on Cancer., 2010), in which a tumor is assigned values for each of three criteria: Tumor size (T), presence in lymph nodes (N), and metastasis (M):
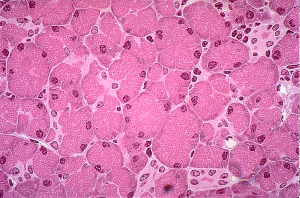
Stage 0: Tis, N0, M0: Carcinoma in situ – neoplastic but not necessarily cancerous cells present in the pancreas; also known as pre-cancer. No cancerous cells detected in lymph nodes (N of 0) or elsewhere (M of 0).
Stage I: Broken into two substages:
IA: T1, N0, M0: Tumor of less than 2cm in its largest dimension in the pancreas; no cancerous cells in lymph nodes or elsewhere
IB: T2, N0, M0: Tumor of greater than 2cm in its largest dimension in the pancreas; no cancerous cells in lymph nodes or elsewhere
Stage II: Broken into two substages:
IIA: T3, N0, M0: Tumor extends beyond the boundaries of the pancreas but does not impinge the celiac axis or superior mesenteric artery; no cancerous cells in lymph nodes or elsewhere
IIB: T1-3, N1, M0: Tumor size may be T1 through T3 as defined above but includes presence of cancerous cells in the regional lymph node (local metastasis).
Stage III: T4, Nx, M0: Tumor involves the celic axis or superior mesenteric artery but does not include distal metastasis; may include spread to regional lymph nodes.
Stage IV: Tx, Nx, M1: Tumor may be of any size or lymph node distribution as defined above, but has metastasized to distal tissues.
The importance of staging, beyond establishing a physical description of the size and location of the tumor cells, is unclear as state-of-the-art treatment has not shown any significant impact on survival (Hidalgo et al., 2014).
Diagnosis and Treatment
Initial diagnosis of pancreatic cancer occurs with a basic external physical exam, which can detect physical masses (e.g. tumor masses or an enlarged liver if the cancer has spread) or fluid build-up in the abdomen. Yellow skin or eyes are indicative of jaundice, which may be another initial warning sign, as are enlarged lymph nodes. Detection of symptoms such as these indicate further testing.
Blood Tests
Several types of blood tests may be used to help diagnose pancreatic cancer:
- Liver Functions: in individuals with jaundice, tests that look at bilirubin levels may be used to determine whether the jaundice is liver related; if not it may indicate a blockage of bile flow, which may indicate a tumor.
- Tumor Markers: CA 19-9 and CEA are both markers that are released in advanced cases of pancreatic cancer, but neither is reliable enough to be definitive without additional tests.
- Pancreatic Hormone Levels: Blood levels of pancreatic hormones such as insulin, glucose, C-peptide, gastrin, glucagon, somatostatin, pancreatic polypeptide, and vasoactive intestinal peptide may be elevated in cases of pancreatic cancer. Again, since there are other reasons why these hormone levels may be higher than expected, these blood tests are not in and of themselves definitive of pancreatic cancer.
The frequency of blood draws from cancer patients makes it relatively easy to prospectively collect blood-derived pancreatic cancer samples such as serum and plasma, but scientists need to be realistic about volumes, custom protocols or anything else that deviates from standard care protocols. The more your collection requirements deviate from standard care, the harder the request will be to fill.
Imaging Tests
A variety of imaging tests may be used to diagnose and stage pancreatic cancer. These are generally very reliable, as they are able to see through the dense abdominal tissues surrounding the pancreas. These include general tissue-scan systems such as CT, MRI, and PET scans. More targeted approaches include cholangiopancreatographies (endoscopic, MRI, and x-ray based), which are able to specifically image the pancreas. Endoscopic cholangiopancreatography utilizes x-ray contrast dyes to highlight the bile ducts in the pancreas.
Somatostatin Receptor Scintigraphy is a specialized type of imaging used to detect several different forms of cancer, including pancreatic cancer. Radiolabelled ocreotide (a somatostatin analog) is injected intravenously and attaches to tumor cells, which tend to express Somatostatin receptors at relatively high levels (sun coy curr drug deliv). Imaging is then performed using a large-field-of-view gamma camera (society of nuclear medicine proc 2002).
Another specialized form of medical imaging commonly used to detect and stage pancreatic cancer is angiography (also known as arteriograpy). Angiography utilizes a radio-opaque dye, which is injected into the celiac and superior mesenteric arteries to ensure that the pancreatic vessels are fully permeated. X-ray fluoroscopy is then used to detect the structure of the blood vessels within the pancreas, which can indicate the severity of pancreatic lesions.
Biopsy
While blood and imaging tests can suggest the presence of pancreatic cancer, only a tissue biopsy followed by microscopy can conclusively diagnose this disease. Fine-needle aspiration (FNA) or endoscopic ultrasound-guided biopsies are performed under imaging guidance, allowing a clinician to capture samples directly from the tissue mass. Endoscopic cholangiopancreatography (one of the imaging methods described above) can also be combined with a physical collection of tissue, as can an endoscopic ultrasound.
From: De La Cruz et al., 2014; Hanada et al., 2014; Yamaguchi et al., 2014
Treatment
Treatment of pancreatic cancer varies depending on the severity and stage of the disease. Standard treatments include surgical or ablative removal of cancerous tissues, radiotherapy, and chemotherapy.
Typically if the cancer has not spread beyond the pancreas, it is considered resectable and surgical removal is the preferred treatment. Depending on the location of the tumor, a pancreaticoduodenectomy or distal pancreatectomy is performed. In some cases it may be discovered during surgery that complete removal is not possible. In either case, since pancreatic cancer has a high frequency of recurrence, additional post-surgical chemo or radiotherapy is prescribed. Because of this, it is common to begin chemo or radio therapies before surgery if the patient is healthy enough.
If the cancer has spread, surgery is typically bypassed and chemo and radiotherapies are used exclusively.
The most common drug used in cases of pancreatic cancer is gemcitabine (Gemzar). Frequently this is part of a multi-drug therapy including paclitaxel (Abraxane), erlotinib (Tarceva), or capecitabine (Xeloda). Alternately a cocktail of drugs, FOLFIRINOX, may be used. FOLFIRINOX combines 5-FU, leucovorin, irinotecan (Camptosar), and oxaliplatin (Eloxatin). While FOLFIRINOX has been shown to extend the lifespan of patients with pancreatic cancer, it has more severe side effects, and as such may be limited to individuals who are otherwise healthy (Tourkantonis, Peponi, Syrigos, & Saif, 2014).
Looking Forward
There has been some promising research using genetically engineered viruses in the treatment of pancreatic cancer. Oncolytic virotherapy takes advantage of the strong target specificity of certain viruses to deliver these engineered viruses directly to the tumor cells. Most recently a study using a modified form of the Vaccinia virus containing IL-10 demonstrated a long-term protection against recurrence of pancreatic cancer (a common problem) in a mouse model (Chard et al., 2014), and resulted in an increased frequency of tumor-specific T cells. While no human trials have been scheduled, virotherapies in general, and this modified Vaccinia virus, show great promise in the future treatment of this fatal disease.
Considerations for Researchers
Given that the only truly definitive diagnostic for pancreatic cancer is biopsy, and that surgical resection has been shown to offer a significantly improved prognosis for patients at any stage of pancreatic cancer (regardless of whether or not they will also be receiving radio or chemotherapy), the availability of pancreatic cancer samples and tissues should be generally good, especially as biopsy or fine needle aspirate (FNA) samples. A recent survey of two hospitals (The University of Erlangen in Germany and the UCLA Medical Center in Los Angeles) showed that resections had been performed of all sizes (from 1 cm in diameter to greater than 5 cm in diameter). However, as mentioned above, pancreatic cancer is rarely detected until the cancer is fairly advanced, and as such the availability of early-stage tissue samples may be limited. Further, the recent trends in cancer is to attempt treatment prior to surgery making biospecimens that are treatment-free even more scarce.
For pancreatic cancer samples collected prospectively, plan ahead as collection times can exceed several months depending on your inclusion and exclusion criteria. Biomarker information is only available if it was collected as part of standard care at the time of treatment. Data mining fees may apply if you require specific biomarker criteria. Remember that many biorepositories and biobanks contain primarily FFPE pancreatic cancer samples that are “CAP graduated” archival samples, which means that they are at least ten years old, and data associated with the samples will reflect standard care as it was a decade or more ago.
Biomarker screening of pancreatic cancer samples is possible, but this is unlikely to be cost effective. If you only want pancreatic cancer samples with certain markers, remember that you will have to pay for testing of the ones that are negative too. A better alternative may be to purchase sections only for a statistically suitable number of cases, screen them in your own lab using your preferred assay or third party, high volume genetic screening service, then request the blocks of interest.
References
Chard, L., Maniati, E., Wang, P., Zhang, Z., Gao, D., Wang, J., . . . Wang, Y. (2014). A Vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-14-0464
De La Cruz, M. S., Young, A. P., & Ruffin, M. T. (2014). Diagnosis and management of pancreatic cancer. Am Fam Physician, 89(8), 626-632.
Edderkaoui, M., & Eibl, G. (2014). Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol, 5, 490. doi: 10.3389/fphys.2014.00490
Edge, S. B., & American Joint Committee on Cancer. (2010). AJCC cancer staging manual (7th ed.). New York: Springer.
Hanada, K., Okazaki, A., Hirano, N., Izumi, Y., Teraoka, Y., Ikemoto, J., . . . Yonehara, S. (2014). Diagnostic strategies for early pancreatic cancer. J Gastroenterol. doi: 10.1007/s00535-014-1026-z
Hidalgo, M., Cascinu, S., Kleeff, J., Labianca, R., Lohr, J., Neoptolemos, J., . . . Heinemann, V. (2014). Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology. doi: 10.1016/j.pan.2014.10.001
Rahib, L., Smith, B. D., Aizenberg, R., Rosenzweig, A. B., Fleshman, J. M., & Matrisian, L. M. (2014). Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res, 74(11), 2913-2921. doi: 10.1158/0008-5472.CAN-14-0155
Ryan, D. P., Hong, T. S., & Bardeesy, N. (2014). Pancreatic adenocarcinoma. N Engl J Med, 371(22), 2140-2141. doi: 10.1056/NEJMc1412266
Tourkantonis, I. S., Peponi, E., Syrigos, K. N., & Saif, M. W. (2014). Pharmacogenetics in pancreatic cancer. JOP, 15(4), 335-339. doi: 10.6092/1590-8577/2689
Yamaguchi, K., Okusaka, T., Shimizu, K., Furuse, J., Ito, Y., Hanada, K., . . . Committee for revision of clinical guidelines for pancreatic cancer of Japan Pancreas, S. (2014). EBM-based Clinical Guidelines for Pancreatic Cancer (2013) issued by the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol, 44(10), 883-888. doi: 10.1093/jjco/hyu127
