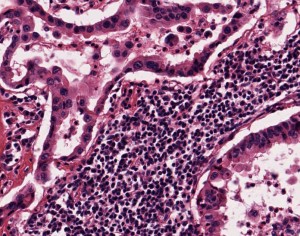
NSCLC samples and other lung cancer samples
Table of Contents
Introduction | Classification | Diagnosis and Treatment | Considerations for Researchers | References
Looking for human lung cancer samples for use in research? Contact us for a consultation.
Introduction
Lung cancer is the most common cancer worldwide and accounts for 1.37 million deaths annually. It is also the leading cause of cancer related mortality in the United States. In 2014 the American Cancer Society estimates 224,210 new cases of lung cancer and an estimated 159,260 deaths, accounting for 27% of all cancer fatalities among both men and women. It mainly occurs in elderly people 60 years of age or older (see http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics ) (See: Table 1).
Table 1: Lung Cancer Incidence Rates
Rates are based on cases diagnosed in 2006-2010 from 18 SEER geographic areas.
| Incidence Rates by Race | ||
| Race/Ethnicity | Male | Female |
| All Races | 74.3 per 100,000 men | 51.9 per 100,000 women |
| White | 74.5 per 100,000 men | 54.6 per 100,000 women |
| Black | 95.8 per 100,000 men | 52.2 per 100,000 women |
| Asian/Pacific Islander | 50.7 per 100,000 men | 28.1 per 100,000 women |
| American Indian/Alaska Native a | 51.2 per 100,000 men | 35.7 per 100,000 women |
| Hispanic b | 40.6 per 100,000 men | 26.3 per 100,000 women |
Classification
There are two major types of Lung cancer indications, namely non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) with NSCLC accounting for 85-90% of all cases.
Non-small cell lung cancer (NSCLC) = 85% to 90% of lung cancers. There are 3 main subtypes of NSCLC that differ in size, shape, and chemical make-up. Subtypes are often grouped together based on similar approaches to treatment and prognosis.
Small cell lung cancer (SCLC): accounts for 10%-15% of lung cancers. SCLC is statistically more strongly correlated with smoking than NSCLC, grows more rapidly, and spreads to other parts of the body more quickly. SCLC is also generally more responsive to chemotherapy.
Other types of lung cancer namely Lung carcinoid tumors: account for fewer than 5% of lung tumors. Most of these tumors grow slowly and rarely spread.
Staging
Staging lung cancer is based on whether the cancer is local or has spread from the lungs to the lymph nodes or other organs. Because the lungs are large, tumors can grow in them for a long time before they are found. Most people with lung cancer are diagnosed at stages III and IV. (See: Table 2)
Table 2 : SEER DATABASE Lung cancer Stage Distribution and 5-year Relative Survival by Stage at Diagnosis (2003-2009) as per the American Cancer Society
| Stage Distribution and 5-year Relative Survival by Stage at Diagnosis for 2003-2009, All Races, Both Sexes |
||
| Stage at Diagnosis | Stage Distribution (%) |
5-year Relative Survival (%) |
| Localized (confined to primary site) | 15 | 53.5 |
| Regional (spread to regional lymph nodes) | 22 | 26.1 |
| Distant (cancer has metastasized) | 57 | 3.9 |
| Unknown (unstaged) | 6 | 7.8 |
Stages of Non-Small Cell Lung Cancer
Stage I: The cancer is located only in the lungs and has not spread to any lymph nodes.
Stage II: The cancer is in the lung and nearby lymph nodes.
Stage III: Cancer is found in the lung and in the lymph nodes in the middle of the chest, also described as locally advanced disease. Stage III has two subtypes:
- If the cancer has spread only to lymph nodes on the same side of the chest where the cancer started, it is called stage IIIA.
- If the cancer has spread to the lymph nodes on the opposite side of the chest, or above the collar bone, it is called stage IIIB.
Stage IV: This is the most advanced stage of lung cancer, and is also described as advanced disease. This is when the cancer has spread to both lungs, to fluid in the area around the lungs, or to another part of the body, such as the liver or other organs.
Key Genes and Mutations
Subsets of NSCLC can be further defined at the molecular level by recurrent ‘driver’ mutations that occur in multiple oncogenes, including AKT1, ALK, BRAF, EGFR, HER2, KRAS, MEK1, MET, NRAS, PIK3CA, RET, and ROS1 (See: Table 3).
Epidermal growth factor receptor (EGFR) gene mutations frequent in adenocarcinoma of the lung in nonsmokers and are implicated in the development of such lesions (http://standardofcare.com/Lung_Cancer)
K-RAS gene mutations have been found in 20–30% of non-small cell lung cancer and also occur most commonly in adenocarcinoma. A meta-analysis based on previously published literature performed by Meng and co-workers (2013) suggested that K-RAS mutations are found in both former/current smokers and non-smokers, but are rarer in the latter and are associated with a worse overall survival, especially in patients with adenocarcinoma and early stage. These mutations which are less common in East Asian vs. US/European patients (Riely et al. 2008; Sun et al. 2010) are missense mutations, which introduce an amino acid substitution at position 12, 13, or 61 resulting in constitutive activation of KRAS signaling pathways thereby defining a distinct molecular subset of the disease. Currently, there are no direct anti-KRAS therapies available.
Table 3 : Frequency of mutations and availability of targeted therapies in NSCLC
| Gene | Alteration | Frequency in NSCLC |
| AKT1 | Mutation | 1% |
| ALK | Rearrangement | 3–7% |
| BRAF | Mutation | 1–3% |
| DDR2 | Mutation | ~4% |
| EGFR | Mutation | 10–35% |
| FGFR1 | Amplification | 20% |
| HER2 | Mutation | 2–4% |
| KRAS | Mutation | 15–25% |
| MEK1 | Mutation | 1% |
| MET* | Amplification | 2–4% |
| NRAS | Mutation | 1% |
| PIK3CA | Mutation | 1–3% |
| PTEN | Mutation | 4–8% |
| RET | Rearrangement | 1% |
| ROS1* | Rearrangement | 1% |
Note : One NSCLC case report with each of these mutations also responded to Crizotinib (Ou et al. 2011) the latter being a partial response (Bergethon et al. 2012)
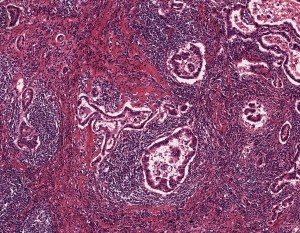
A specimen of non-small cell lung cancer adenocarcinoma, used in histopathology research, at 5x magnification.
Diagnosis and Treatment
Current Treatments/Targeted Therapies
(http://www.lungcancer.org; http://www.standardofcare.com)
The discovery of certain proteins or biomarkers gives oncologists the option of performing tests to determine if one of these biomarkers is present in the tumors thereby helping them to decide which treatment options will work the best. These individualized treatments may help patients avoid treatments that are unlikely to work and help to decrease the side effects of treatment. These markers are found on the cell surface or in the genes that program cells. Some biomarkers include: Epidermal growth factor receptor (EGFR), Anaplastic lymphoma kinase (ALK) and K-ras mutations (KRAS 1). Chemotherapy drugs are used to attack specific targets in cancer cells by attaching to or blocking these targets. People with advanced lung cancer with certain molecular biomarkers could receive treatment with a single targeted drug alone or in combination with chemotherapy. The treatments for lung cancer include:
- Afatinib and Erlotinib (Tarceva): FDA approved Afatinib in July 2013 as a first-line treatment for patients with metastatic NCSLC tumors that have exon 19 deletions or exon 21 (L858R) EGFR substitution mutations (Yu H.A & Pao W 2013 to block EGFR. Erlotinib can be used even in the absence of the mutation for patients in need of additional treatment.
- Bevacizumab (Avastin): Vascular endothelial growth factor (VEGF) stimulates blood vessels to penetrate tumors and supply oxygen, minerals, and other nutrients to feed the tumor just like normal tissues. Tumors spread by releasing VEGF to create new blood vessels. Bevacizumab is targeted to stop VEGF from stimulating the growth of new blood vessels. It has been shown to improve response and overall rate survival in people with advanced NCSLC when used in combination with chemotherapy.
- Crizotinib (Xalkori): Mutations in ALK genes program the way in which cells function, and lead to increased tumor cell growth. Crizotinib works by blocking ALK and stopping the growth of the tumor. Crizotinib, an oral active ALK inhibitor is an approved treatment for patients with advanced-stage ALK-rearranged non-small cell lung cancer and has already shown impressive clinical activity. (Bos et al 2013 and Killock 2014). It has shown 57% overall response rate, 87% disease control rate and 6 month progression free survival of 72% in patients with adenocarcinoma (Bang Y et al).
- Adjuvant cisplatin plus vinorelbine: prolongs disease free and overall survival in patients with completely resected early stage non-small cell lung cancer. First line treatment with chemotherapy regimens have a median survival of 8-10 months, with 1 and 2-year survival rates of 35-45% and 10-20%, respectively.
Trends in Standard Care Looking Forward
Despite caveats, ‘driver’ mutations that may be linked to outcomes with targeted therapies in SCC are emerging. Altered genes include FGFR1 and DDR2 as well as PIK3CA. In addition, results from a recent large genomic study in lung SCC have added a variety of potential therapeutic targets that await validation in prospective clinical trials (Hammerman et al. 2012).
At ASCO 2013 Fabrice Barlesi, MD, PhD, of Aix-Marseille University described a French biomarkers study on the feasibility of NSCLC Tumor Profiling and Biomarker-Guided Therapy. KRAS mutations were found to be most common in smokers (31.7%) whereas EGFR activating mutation was seen most often in never-smokers (33.2%). The project so far includes six markers: EGFR, KRAS, ALK, BRAF, HER2, and PI3K.
Targeted NSCLC treatment using screening for EGFR mutation, which is often linked to Genentech’s/Astellas’s Tarceva’s SATURN (Sequential Tarceva in Unresectable Non-Small Lung Cancer) has been found effective in a maintenance trial in patients with advanced, inoperable, metastatic NSCLC treated with first-line platinum based doublet-responders treated with erlotinib or placebo: greatest benefit in patients with EGFR mutation positive group with a 90% reduction in the risk of disease progression compared to placebo, but only 11% had such a mutation.
- Pfizer’s new therapy Xalkori which is indicated in advanced NSCLC patients with the ALK-positive gene abnormality.
- Anaplastic lymphoma kinase (ALK) mutations and epidermal growth factor receptor (EGFR) are anticipated to remain the only identified intracellular molecular aberrations targeted by marketed products. Instead, the late stage pipeline is mainly occupied by immunotherapeutic procedures which still offer a larger degree of specificity than chemotherapies; however, they initiate the destruction of tumor cells via the immune system. Phase III immunotherapies like Yervoy (ipilimumab), necitumumab and nivolumab are expected to reach the market in the next few years, each with a specific target.
Considerations for Researchers
Surgical resections are often performed for stage I and II lung cancers, so early stage lung cancer samples are likely to be available as FFPE blocks. Biopsies for stage III and IV may be available but are hard to obtain because they are often used by the clinic and may not ever reach the clinic’s repository. The prevalence of the disease and the frequency of blood draws from cancer patients make it relatively easy to obtain FFPE NSCLC samples, blood derived NSCLC samples like plasma and serum, or even “matched pairs” of tumor tissue and biofluids from the same donor, tissue but scientists need to be realistic about requesting volumes greater than 1 ml, custom collection protocols, or anything else that deviates from standard care. The more your collection requirements deviate from standard care, and the the harder the request will be to fill.
For NSCLC samples or SCLC samples collected prospectively, plan ahead as collection times can exceed several months depending on your inclusion and exclusion criteria. Biomarker information is only available if it was collected as part of standard care at the time of treatment. Data mining fees may apply if you require knowledge of specific biomarker criteria that were tested for as part of standard care. Remember that many biorepositories and biobanks contain primarily FFPE samples that have been CAP graduated, which means that elapsed time since the collection procedure often exceeds ten years, and data associated with the samples will reflect standard care as it was a decade ago. Biomarker screening of samples by us is possible, but this is unlikely to be cost effective as you will have to pay for testing of the ones that are negative too. A better alternative may be to purchase sections only for a statistically suitable number of cases, screen them in your own lab using your preferred assay or a third-party, high volume genetic screening service, then request the blocks of interest.
References
- American cancer Society(http://www.cancer.org/cancer/lungcancer/)
- http://www.Lungcancer.org
- Source: SEER Cancer Statistics Review 2013 (http://www.seer.cancer.gov/)
- North American Association of Central Cancer Registries (NAACCR) – National Resource:
http://www.naaccr.org/ - Kim, H. R., Shim, H. S., Chung, J.-H., Lee, Y. J., Hong, Y. K., Rha, S. Y., Kim, S. H., Ha, S.-J., Kim, S. K., Chung, K. Y., Soo, R., Kim, J. H. and Cho, B. C. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer, 118: 729–739. doi: 10.1002/cncr.26311 (2012)
- Meng D ,Yuan M , Li X , et al. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: A systematic review with meta-analysis. Lung Cancer;81(1):1-10 (2013)
- Oua S.I, Bartlett C.H, Mino-Kenudsonc M, et al. Crizotinib for the Treatment of ALK-Rearranged Non-Small Cell Lung Cancer: A Success Story to Usher in the Second Decade of Molecular Targeted Therapy in Oncology The Oncologist 17(11): 1351-1375 , 2012
- Bos, M, Gardizi M,et al.Activated RET and ROS: two new driver mutations in lung adenocarcinoma. Transl Lung cancer Res 2(2):112-121 (2013).
- Killock D (2014) Alternative rearrangements—targeting ROS1 in NSCLC Nature Reviews Clinical Oncology 11, 624 /doi:10.1038/nrclinonc.2014.180
- Yu H.A & Pao W. Targeted therapies: Afatinib—new therapy option for EGFR-mutant lung cancer .Nature Reviews Clinical Oncology 10, 551–552 (2013) doi:10.1038/nrclinonc.2013.154.
- Christine Lovly, M.D., Ph.D., Leora Horn, M.D., M.Sc., William Pao, M.D., Ph.D. (2013) http://www.mycancergenome.org/content/disease/lung-cancer
- Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang YJ, Kim DW, Christensen J, Tan W, Wilner KD, Salgia R, Iafrate AJ (2011) Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011 May;6(5):942-6
- Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ (2012). ROS1 rearrangements define a unique molecular class of lung cancers. ; 30(8):863-70
- Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012 Sep 27;489(7417):519-25. doi: 10.1038/nature11404. Epub 2012 Sep 9.
- http://www.cancer.gov/cancertopics/types/lung
- http://www.cancernetwork.com/conference-reports/asco2013/lung-cancer/content/article/10165/2145686