Breast Cancer Samples
Table of Contents
Introduction | Classification | Diagnosis and Treatment | Considerations for Researchers seeking breast cancer samples | References
Looking for human breast cancer samples for use in research? Contact us for a consultation.
Introduction
Breast cancer (BC) is the number one cause of cancer-related mortality among females worldwide (Torre et al, 2015) and the second leading cause of cancer death in U.S. women, exceeded in the U.S. only by lung cancer (American Cancer Society). Due to the prevalence of this disease, breast cancer samples for use in research are in high demand.
Globally, 25% of all cancer cases diagnosed in women are BC, with more than half of those being located in more-developed countries (Torre et al, 2015). The American Cancer Society estimates just under 250,000 new cases of invasive breast cancer will be diagnosed in the United States in 2016. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, BC accounted for an estimated 14% of all new cancer cases in the U.S. in the year 2015, and an estimated 40,290 deaths in the same year. BC mortality rates in the U.S. have declined since 1989, with greater decreases in mortality found among women under the age of 50. This decrease is primarily due to earlier detection through screening, improved treatment options and an increase in education and awareness among the affected population (American Cancer Society).
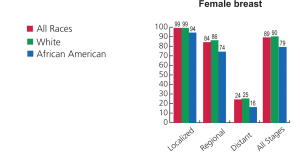
Breast Cancer Statistics for Females –
Ref: Seigel R et al (2014). Cancer Statistics. CA Cancer J Clinic CA, 64:9-29
Lifestyle-related factors and breast cancer risk
- Age of delivery of first child: A slightly higher risk of breast cancer is found in women who have not had any children or women who had their first child after age 30. Pregnancy at a younger age and women who have had multiple pregnancies reduces the risk of breast cancers. An exception to this is Triple-Negative Breast Cancer (TNBC) for which pregnancy increases the risk of disease morbidity.
- Oral contraceptives: Many oral contraceptives have been linked to have been found to have an increased risk of breast cancer.
- Hormone therapy after menopause: Using combined hormone therapy after menopause for current and recent users (within 5 years of stopping combined treatment) increases the likelihood of breast cancer being found at an advanced stage and dying from it. The use of Estrogen therapy (ET) alone does not appear to increase the risk of developing breast cancer.
- Breastfeeding: for 1.5 to 2 years has been suggested to be a slightly lower risk of breast cancer.
- Alcohol use: Alcohol Consumption has been shown to be linked to an increased risk of developing breast cancer.
- Obesity: Obesity post menopause, increases risk of breast cancer.
- Physical activity: 1.25 to 2.5 hours of brisk walking reduces risk of breast cancer by 18% and walking 10 hours per week reduces the risk even further.
Classification
Histological classification of Breast Cancer
Breast cancers can be classified based on their histopathology which refers to the examination of a biopsy or surgical specimen by a pathologist, after the specimen has been processed and histological sections have been placed onto glass slides. Pathology grading systems classify the microscopic cell appearance, abnormality and deviations in their rate of growth with the goal of predicting developments at tissue level. Pathologists describe cells as well differentiated (grade I or low grade), moderately differentiated (grade II or intermediate grade), and poorly differentiated (grade III or high grade) as the cells progressively lose the features seen in normal breast cells. Poorly differentiated cancers have the worst prognosis.
The three most common histo-pathological types collectively represent approximately three-quarters of breast cancers:
- Invasive or infiltrating ductal carcinoma (IDC) – is the most common type of breast cancer and accounts for 55% of breast cancer cases. About 8 of 10 invasive breast cancers are infiltrating ductal carcinomas. It can metastasize to other parts of the body
- Invasive or infiltrating lobular carcinoma (ILC) – is a less common invasive cancer and accounts for 5% of breast cancer cases. About 1 in 10 invasive breast cancer is an ILC. Like IDC it can also metastasize.
- Ductal carcinoma in situ (DCIS) also known as intraductal carcinoma – is the most common type of non-invasive breast cancer and accounts for 13% of all breast cancer cases. About 1 in 5 new breast cancer cases are DCIS with it being curable for almost all the women diagnosed.
Special types of invasive breast carcinoma
There are some special types of breast cancer which are sub-types of invasive carcinoma named based on their histo-pathological features.
- Sub-types with the same or maybe worse prognosis than standard infiltrating ductal carcinoma. These include: Metaplastic carcinoma (most types, including spindle cell and squamous), Micropapillary carcinoma and Mixed carcinoma (has features of both invasive ductal and lobular)
- Less common types of breast cancer with a bad prognosis:
- Inflammatory breast cancer (in its initial stages often mistaken for an infection known as mastitis) accounting for 1-3% of breast cancers and has a higher chance of spreading and a worse prognosis than typical invasive ductal or lobular cancer.
- Paget disease of the nipple- almost always associated with either ductal carcinoma in situ (DCIS) or infiltrating ductal carcinoma. The prognosis is good if the biopsy shows DCIS but no invasive cancer. The prognosis is not as good if invasive cancer is observed.
- Phyllodes tumor and Angiosarcoma.
- Subtypes with a better prognosis than standard infiltrating ductal carcinoma. These include: Adenoid cystic (or adenocystic) carcinoma, Low-grade adenosquamous carcinoma, Medullary carcinoma, Mucinous (or colloid) carcinoma, Papillary carcinoma and Tubular carcinoma.
Classification based on stage
The TNM classification for staging breast cancer is based on the size of the cancer where it originally started in the body and the locations to which it has traveled.
Classification based on receptor status
Several genes and cell receptors are key for diagnosis, classification and treatment of breast cancer ER, PR and HER2 receptor status. Breast cancer can also be classified based on proteins on or in the cancer cells, into groups like hormone receptor-positive or triple-negative. Estrogen and progesterone hormones often fuel the growth of breast cancer cells. Breast cancers referred to as ER-positive or ER+ and/or PR-positive or PR+ have estrogen and/or progesterone. The epidermal growth factor receptor (EGFR) is the founding member of the ErbB family of four structurally related receptor tyrosine kinases. In humans this includes HER1 (EGFR, ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4). Each receptor can be either positive or negative i.e. ER positive (ER+), ER negative (ER-), PR positive (PR+), PR negative (PR-), HER2 positive (HER2+), and HER2 negative (HER2-).
Hormone positive breast cancers can be treated with drugs that lower hormone levels or block estrogen receptor (ER), progesterone receptor (PR), and HER2/Neu. These cancers are slower growing than hormone negative cancers.
Cells negative for all of these receptors, i.e. ER-, PR- and HER2-, are called basal-like or triple negative breast cancer (TNBC). TNBC tend to spread and grow more quickly than other types of breast cancer. TNBC is an extremely aggressive type of breast cancer which is not responsive to currently available targeted treatments like tamoxifen and or trastuzumab. Breast cancers with these characteristics tend to occur more often in younger women and in African- or Hispanic/Latino ethnicity.
Activation of Androgen receptor (AR) is expressed in 80-90% of ER+ breast cancers and 40% of “TNBC” breast cancers. AR appears to suppress breast cancer growth in ER+ cancer while in it appears to act as growth promoter in ER- breast cancer and is being investigated as prognostic marker for treatment. (Lehman et al, 2011)
DNA-based classification
(http://ghr.nlm.nih.gov/condition/breast-cancer)
Specific DNA mutations or gene expression profiles identified in the cancer cells may guide the selection of treatment. The most prominent genetic profiles based on clinical evidence that have been developed for breast cancer are:
Inherited gene mutations
Inherited mutations in genes like BRCA1 and BRCA2 cluster in families and are associated with breast and ovarian cancer. Men with BRCA1 mutations also have an increased risk of developing breast cancer. Genetic testing can identify some women who have inherited mutations in the BRCA1 or BRCA2 tumor suppressor genes. Variations of the BRCA1, BRCA2, CDH1, STK11, and TP53 genes increase the risk of developing breast cancer. Inherited changes in several other genes, including CDH1, STK11, and TP53, have been found to increase the risk of developing breast cancer. Inherited variants of the ATM, BARD1, BRIP1, CHEK2, NBN, PALB2, RAD50, and RAD51 genes, as well as certain versions of the AR gene have been implicated by research studies to be associated with breast cancer risk.
Somatic gene mutations
Somatic mutations are not inherited but acquired in certain cells and built up during a person’s life time. Somatic mutations in AR, ATM, BARD1, BRIP1, CHEK2, DIRAS3, ERBB2, NBN, PALB2, RAD50, and RAD51 genes are associated with breast cancer. ATM and CHEK2 have the strongest evidence of being related to the risk of developing breast cancer. Somatic mutations in the ERBB2 (also called Her-2/neu), DIRAS3, and TP53 genes have been associated with some cases of breast cancer.
Classifying breast cancer based on gene expression
Parise and Caggiano (2014) recently assessed survival of patients using ER/PR/HER2 and illustrated the heterogeneity of HER2+ subtypes thereby providing a clear separation in survival and adjusted mortality. They compared the usefulness of the ER/PR/HER2 subtyping of breast cancer with an IHC surrogate classification. They found that ER/PR/HER2 subtype testing was a simple, inexpensive, easy to interpret, reliable, reproducible, and readily available for clinicians without additional tests. They also found that the heterogeneity of the HER2+ subtypes and the prognostic importance of ER and PR expression was obvious when using ER, PR, and HER2 alone. Gene expression profiling studies correlated with immuno-histochemical biomarkers (Brown et al 2004; Bauer et al 2010) and identified four categories of breast cancer, namely luminal A, luminal B, HER2 overexpressing, and basal-like or triple negative breast cancer (Sørlie et al , 2001).
Diagnosis and Treatment
Researchers have looked at different gene expression profiles and found that they can help predict if an early stage breast cancer is likely to recur after initial treatment. Several such tests, which look at different sets of genes, are now available:
- Oncotype DX®. The Oncotype DX test is used for patients with ductal carcinoma in situ (DCIS)/small tumors or invasive carcinoma that have not spread to lymph nodes for women with early stage, hormone receptor positive breast cancers. This could be helpful in deciding whether adjuvant treatment with chemotherapy (after surgery) might be useful. http://breast-cancer.oncotypedx.com/en-US
- MammaPrint® is the first and only FDA-cleared IVDMIA breast cancer recurrence assay and can be used to help determine how likely breast cancers are to recur in a distant part of the body after initial treatment. MammaPrint® gene pattern is a commercial-stage 70-gene panel marketed by Agendia and categorizes tumors as either high or low risk. http://www.agendia.com/pages/mammaprint/21.php
- The THEROS Breast Cancer Index® (BCI) is a combination of biomarkers that improve risk stratification and treatment outcome prediction in patients with estrogen receptor (ER)-positive, lymph node-negative breast cancer. The THEROS BCI is a tool that can help oncologists and patients make information-based decisions about breast cancer therapy. http://www.prnewswire.com/news-releases/data-from-three-clinical-studies-of-biotheranostics-breast-cancer-molecular-test-presented-at-ctrc-aacr-san-antonio-breast-cancer-symposium-79136317.html
- Mapquant Dx HR : Ipsogen announced the European launch of the Mapquant Dx HR test for measuring the expression of genes related to estrogen and progesterone. http://www.laboratorytalk.com/analytical-instruments/clinical-and-lifescience-analysis/clinical-chemistry-analysis/ipsogen-launches-mapquant-dx-hr-test-in-europe/340018.article
- Receptor status is a critical assessment for all breast cancers as it determines the suitability of using targeted treatments such as tamoxifen and or trastuzumab. These treatments are now some of the most effective adjuvant treatments of breast cancer. On the other hand to date ‘triple negative breast cancer (TBNC)” lacks targeted treatments and has a poor prognosis compared to the breast cancers with a positive receptor status.
- Circulating tumor cells: have been found in the blood of women with breast cancer and can be detected with lab tests. These can be used to test for the recurrence of the disease and determine if treatments are successful.
Targeted therapies
Prognosis and treatment decisions are still guided by tumor stage, hormone receptor status, HER2/neu status and BRCA mutations. Targeted therapies are a group of newer drugs that specifically take advantage of gene changes in cells that cause cancer.
- PARP inhibitors; are a new class of drugs that have shown promise in clinical trials in patients with BRCA mutation caused cancers that have spread and are resistant to treatment.
- Drugs that target Estrogen receptor positive (ER+) cancer cells: ER+ cancer cells depend on estrogen for their growth, so they can be treated with drugs to reduce either the effect of estrogen (e.g. tamoxifen) or the actual level of estrogen (e.g. aromatase inhibitors), and generally have a better prognosis. Everolimus (Afinitor) is one of the targeted therapy drug approved to be given with Exemestane (Aromasin) to treat advanced hormone receptor-positive breast cancer in post-menopausal women. When added to tamoxifen it has been shown to help in treating advanced hormone receptor-positive breast cancer.
- Drugs that target HER2: Trastuzumab (Herceptin) pertuzumab (Perjeta), ado-trastuzumab emtansine (Kadcyla), and lapatinib (Tykerb) are the drugs currently being used to target HER2.
- Anti-angiogenesis drugs: Angiogenesis is the process by which cancer cells develop blood vessels to nourish cancer cells to grow. Examining angiogenesis can help predict prognosis in breast cancer, as some studies have indicated that specimens surrounded by new blood vessels could be more aggressive. There are several clinical trials anti-angiogenesis drugs in progress.
- Bisphosphonate drugs: include pamidronate (Aredia) and zoledronic acid (Zometa), and are being used to help strengthen bones that are weakened by metastatic breast cancer and reduce a risk of bone fractures. The data obtained so far does not support the use of these drugs for standard treatment.
- Denosumab (Xgeva, Prolia): is also used to reduce bone fracture risk as described above. It is being studied to see if it can help adjuvant treatments work better.
- Vitamin D: Women with early stage breast cancer with vitamin D deficiency have been found to have a poorer prognosis and have metastatic recurrence of cancer as shown in a recent study.
Considerations for Researchers
Traditionally, surgical resections are often performed for stage I and II cancers, so these samples are likely to be available as FFPE blocks. However, if you are searching for samples collected prior to treatment, please note that recent trends have moved towards treatment prior to surgery. Biopsies for stage III and IV may be available but are hard to obtain because they are often used by the clinic and may not ever reach a repository. Blood products may be available on a prospective basis.
For breast cancer samples collected prospectively, plan ahead as collection times can exceed several months depending on your inclusion and exclusion criteria. Biomarker information is only available if it was collected as part of standard care at the time of treatment. Data mining fees may apply if you require specific biomarker criteria. Remember that many biorepositories and biobanks contain primarily FFPE breast cancer samples that have been CAP graduated, which means that they are at least ten years old, and data associated with the samples will reflect standard care as it was a decade or more ago.
The frequency of blood draws from cancer patients makes it relatively easy to prospectively collect blood derived breast cancer samples like plasma and serum, but scientists need to be realistic about requesting volumes greater than 1 ml, custom collection protocols, or anything else that deviates from standard care. The more your collection requirements deviate from standard care, the harder the request will be to fill.
Biomarker screening of samples is possible, but this is unlikely to be cost effective unless you want to purchase all tested specimens regardless of their biomarker profile. If you only want samples with certain markers, expect that you will have to pay for testing of the ones that are negative too. A better alternative may be to purchase sections only for a suitable number of cases, screen them in your own lab using your preferred assay or third party, high volume genetic screening service, then request the blocks of interest.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015), Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65: 87–108. doi: 10.3322/caac.21262 Epub 2015 Feb 4.
- American cancer Society (http://www.cancer.org/cancer/breastcancer/)
- National Cancer Society SEER Fact Sheet (http://seer.cancer.gov/statfacts/html/breast.html/)
- Siegel R; Ma J, Zou Z and Jemal A (2014) Cancer Statistics 2014. CA Cancer J Clin; 64:9-29.
- Parise C.A and Caggiano V (2014) Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immuno-histochemical Biomarkers. Journal of Cancer Epidemiology. Vol 2014 Article ID 469251, http://dx.doi.org/10.1155/2014/469251
- Brown M, Tsodikov A, Bauer K.R , C. Parise A., and Caggiano V (2008), “The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: The California Cancer Registry, 1999–2004,” Cancer112 (4),737–747.
- Bauer K, Parise C, and Caggiano V (2010), “Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen consensus statement for early breast cancer,” BMC Cancer 10: article 228.
- Sørlie T, Perou C.M, Tibshirani R et al (2001) “Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications,” PNAS (USA), 98(19), 10869–10874.
- North American Association of Central Cancer Registries (NAACCR) – National Resource: http://www.naaccr.org/
- http://www.forbes.com/sites/daviddisalvo/2013/02/22/targeted-breast-cancer-drug-ushers-in-a-new-era-of-cancer-treatment/
- Karmanos Cancer Center (http://ww5.komen.org/BreastCancer/SubtypesofBreastCancer.html)
- Davidson N.E (2013).Fifteen Years of Anti-HER2 Therapy. Oncology 7(3):1-2
- Azvolinsky A (2013) Trastuzumab May Have Role in HER2-Negative Breast Cancer Treatment (http://www.cancernetwork.com/her2-positive-breast-cancer/content/article/10165/2132661)
- Jelovac D (2013). HER2-Directed Therapy for Metastatic Breast Cancer. (http://www.cancernetwork.com/her2-positive-breast-cancer/content/article/10165/2132123)
- Eheman CR, Shaw KM, Ryerson AB, Miller JW, Ajani UA, White MC (2009). “The changing incidence of in situ and invasive ductal and lobular breast carcinomas: United States, 1999–2004”. Cancer Epidemiol. Biomarkers Prev. 18 (6): 1763–9. doi:10.1158/1055-9965.EPI-08-1082. PMID 19454615.
- Arpino G, Bardou VJ, Clark GM, Elledge RM (2004). “Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome”. Breast Cancer Res. 6 (3): R149–56. Doi: 10.1186/bcr767. PMC 400666. PMID 15084238.
- Tice JA. (2010).The 70-Gene Signature (Mamma Print) as a Guide for the Management of Early Stage Breast Cancer. California Technology Assessment Forum. 2010 June 2nd (http://www.ctaf.org/content/assessments/detail/?id=1178).
- http://www.agendia.com/pages/mammaprint/21.php
- http://www.prnewswire.com/news-releases/data-from-three-clinical-studies-of-biotheranostics-breast-cancer-molecular-test-presented-at-ctrc-aacr-san-antonio-breast-cancer-symposium-79136317.html
- http://www.laboratorytalk.com/analytical-instruments/clinical-and-lifescience-analysis/clinical-chemistry-analysis/ipsogen-launches-mapquant-dx-hr-test-in-europe/340018.article
- Jeremy H. Howick (2011-02-23). The Philosophy of Evidence-based Medicine. John Wiley & Sons. p. 15. ISBN 978-1-4443-4266-6.
- Lehmann, B. D.; Bauer, J. A.; Chen, X.; Sanders, M. E.; Chakravarthy, A. B.; Shyr, Y.; Pietenpol, J. A. (2011). “Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies”. Journal of Clinical Investigation 121 (7): 2750–2767.Doi:10.1172/JCI45014. PMC 3127435. PMID 21633166